For H(2) gas, the compressibility factor,Z = PV //n RT is
$ 26.50 · 4.9 (454) · In stock

For H(2) gas, the compressibility factor,Z = PV //n RT is
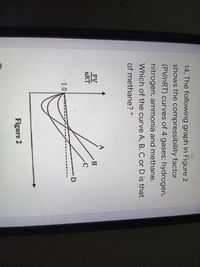
Answered: 14, The following graph in Figure 2…

For H(2) gas, the compressibility factor,Z = PV //n RT is

Solved] The compressibility factor for an ideal gas is

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0-b) = RT may be written as (P+*}() =RT of PV + 9 =RT of PV=RT - For large V (at very

For an ideal gas, the value of compressibility factor `Z(=(pVm)/(RT))` is

SOLVED: Hey guys, please help me friends. Choose the correct answer, don't say wrong answers. Select the correct statement/s regarding the compressibility factor Z of a gas: Z for an ideal gas

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1
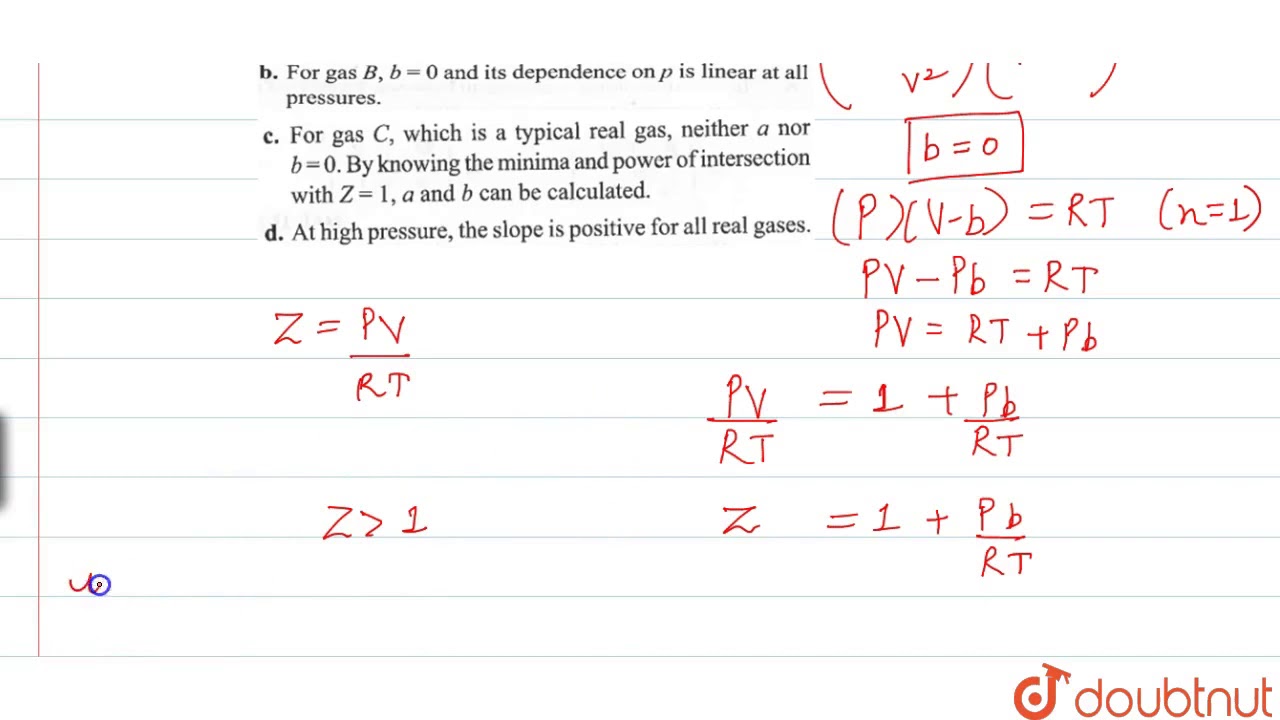
The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `

SOLUTION: State of matter gases liquids and solids - Studypool

Deviation of Real Gases from Ideal Gas Behaviour - Chemistry for ACT PDF Download