Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage
$ 16.00 · 4.5 (473) · In stock
Frontiers Binding mechanism of full-length Aβ40 peptide to a

Journal of Cellular Biochemistry

The amyloid-inhibiting NCAM-PrP peptide targets Aβ peptide

Effects of the Hydrophilic N-Terminal Region on Aβ-Mediated

Simulation model of Aβ42 pore-forming oligomers with the 6RHY fold

Structural architecture of amyloid-β oligomers, curvilinear

Single-molecule Mapping of Amyloid-β Oligomer Insertion into Lipid

Structural details of amyloid β oligomers in complex with human
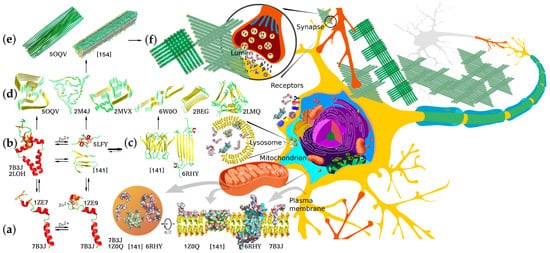
Aβ-Peptide Production and Conformational Behavior

Molecular dynamics simulations reveal the importance of amyloid
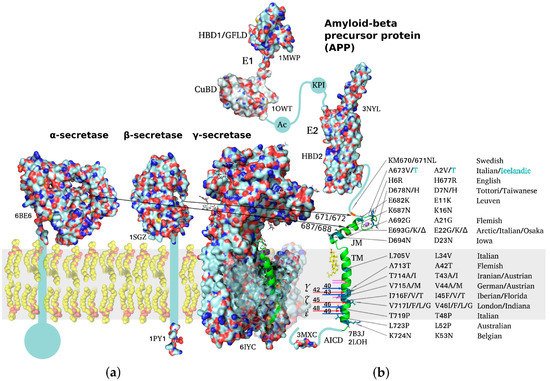
Aβ-Peptide Production and Conformational Behavior

Purity and identity of [U-15 N] Aβ42 and [U-2 H, 13 C, 15 N] Aβ42

Aβ(1-42) tetramer and octamer structures reveal edge conductivity

Structure of AqpZ tetramer and location of mutations. The