What is the compressibility factor (Z) for 0.02 mole of a van der
$ 20.00 · 4.6 (79) · In stock

Resorcinol, C6H6O2
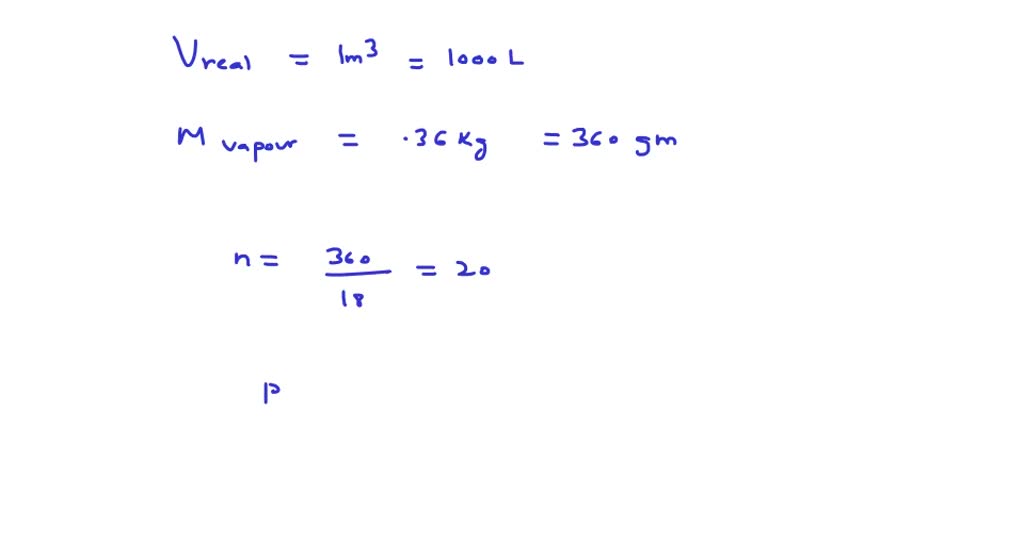
⏩SOLVED:The value of compressibility factor (Z) for this vapour is?…
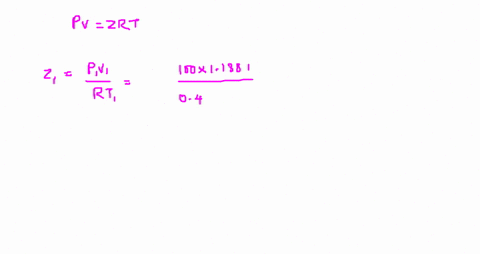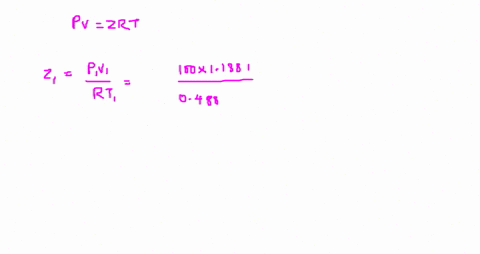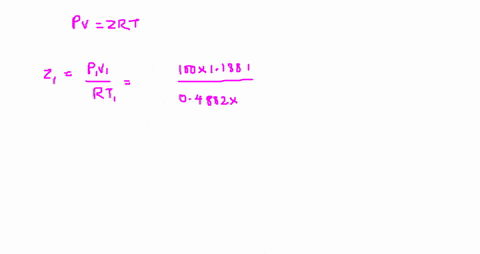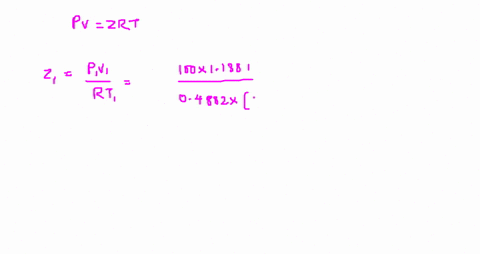
⏩SOLVED:The value of compressibility factor (Z) for this vapour is?…

Solved We begin by showing that the compressibility factor

Van Der Waals Equation - an overview

What is the compressibility factor (Z) for 0.02 mole of a van der Waal
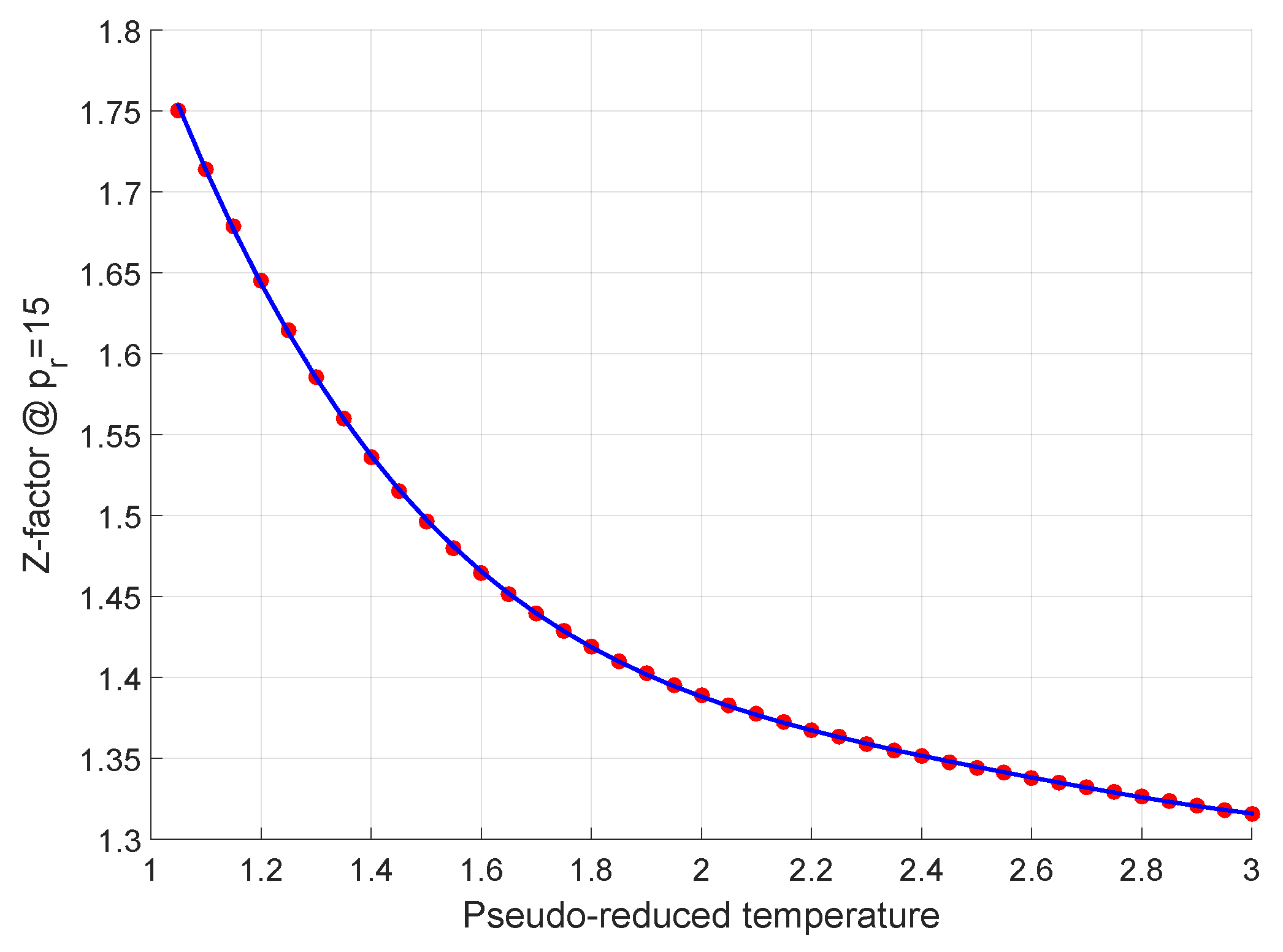
Energies, Free Full-Text

The compression factor (compressibility factor) for 1 mol of a van der
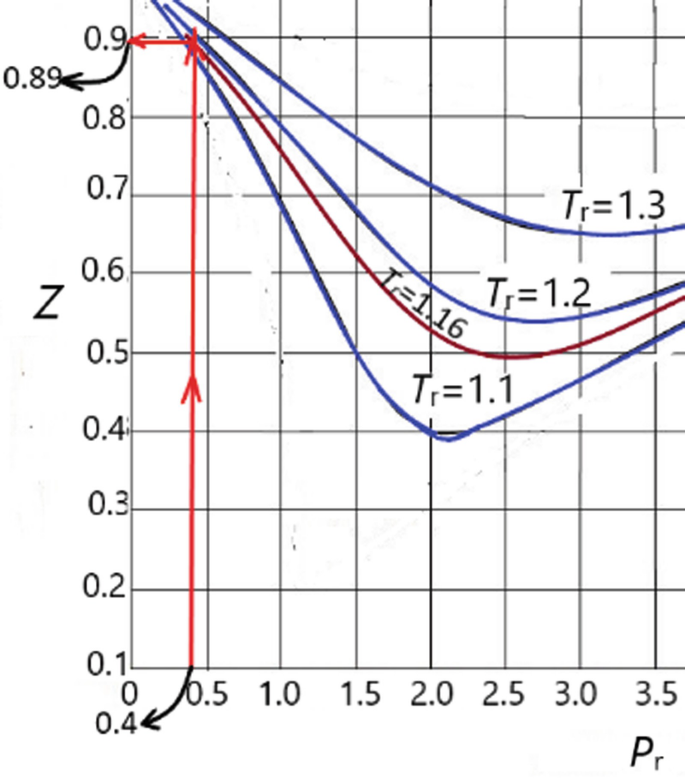
Chemical Thermodynamics
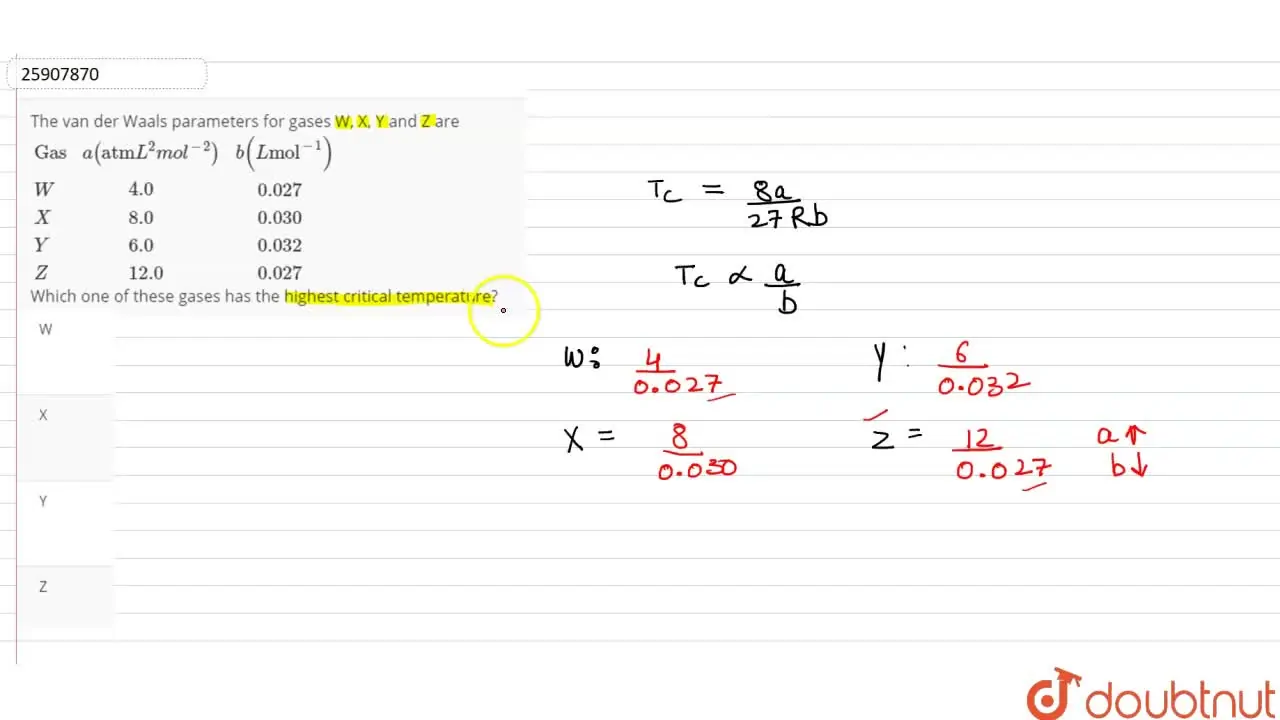
The van der Waals parameters for gases W, X, Y and Z are {:(Gas,a(
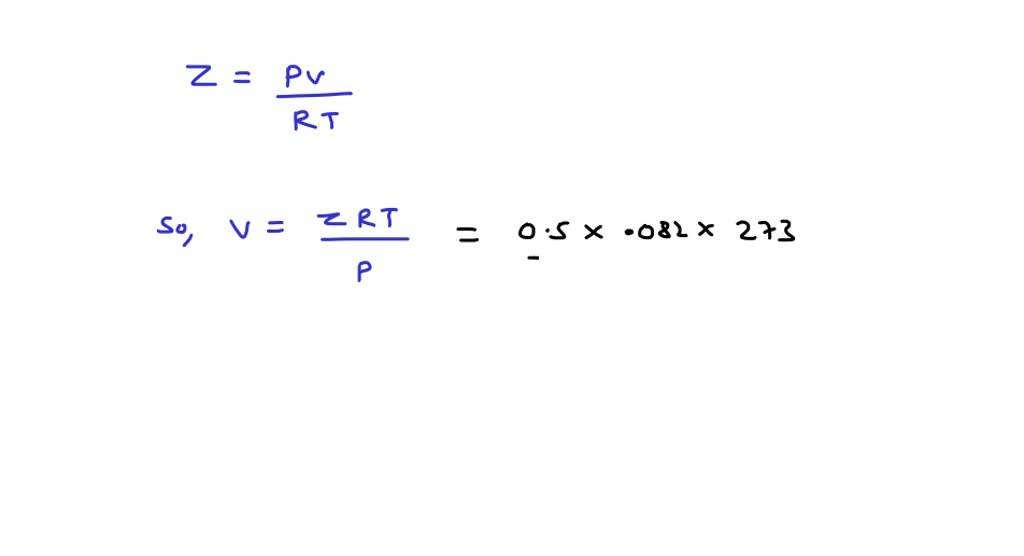
⏩SOLVED:Compressibility factor for 1 mol of a van der Waals gas at…
58.7 Maximum mass of hydrogen is present in(1) 0.1 mol of CH1206(2) 1.5 mol of NH3(3) 22.4 L of H2S(g) at S.T.PSo(4) 0.5 g molecule of CeH

At `2173K` temp, and 9 atm pressure, the compressibility fog a gas is `0.9`. The volume of 1 mill-mo
Soil water diffusivity and water content distribution during outflow experiment

Physical Chemistry The Compression Factor (Z) [w/1 example]
