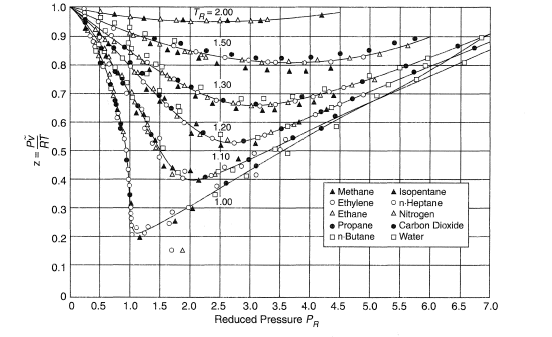
Compressibility factor (Z) for a van der Waals real gas at
$ 13.99 · 4.7 (386) · In stock

Share your videos with friends, family and the world
GAS LAW
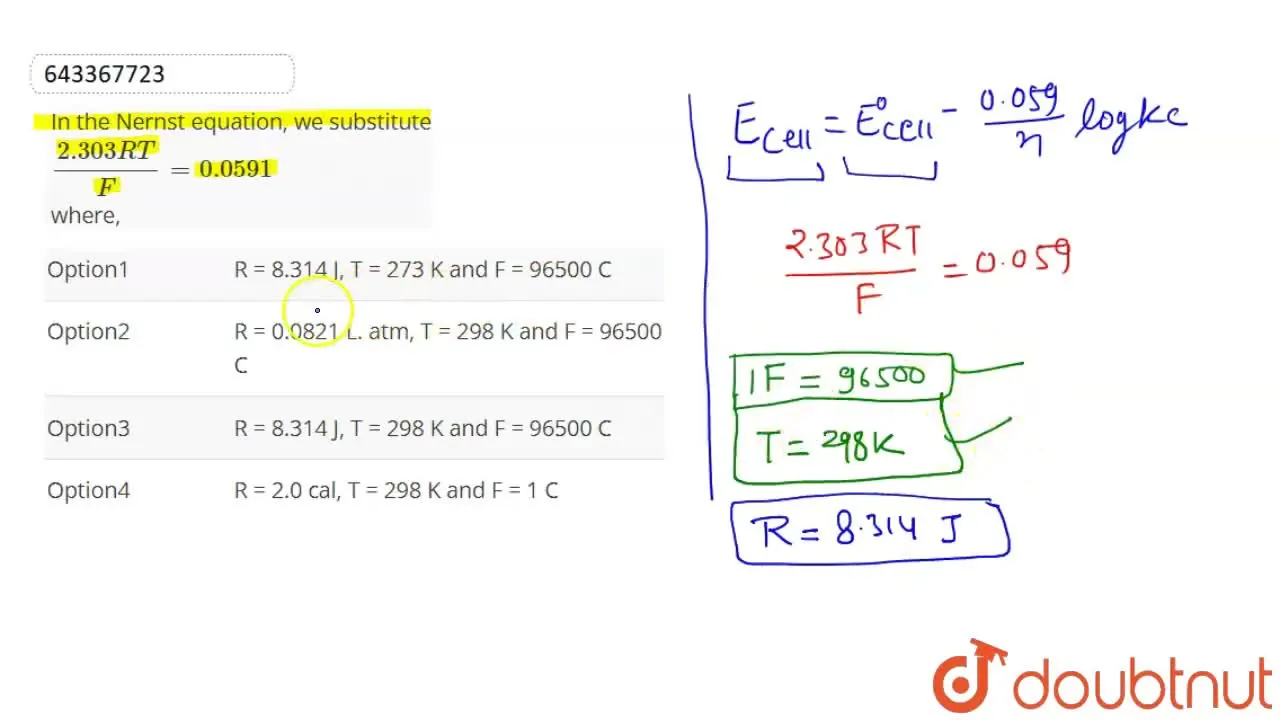
R = 8.314 J, T = 298 K and F = 96500 C

Description of real gases: Compression factor

The compressibility factor Z of one mole of Vander Waals gas with negligible 'a' value is a) bp/RT b) [1-(bp/RT) c)[1 (bp/RT) d) (1/bp)? - EduRev NEET Question

Maxwell's speed distribution curve

Solved Show that the compressibility factor of van der Waals

Van der Waals equation - Wikipedia

Real Gases and the van der Waals Equation Explained

Bengali] What will the value of compressibility factor (Z) be for a g

For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

Compressibility Factor Calculator - File Exchange - MATLAB Central
Lecture 4-Real-Gases, PDF, Gases
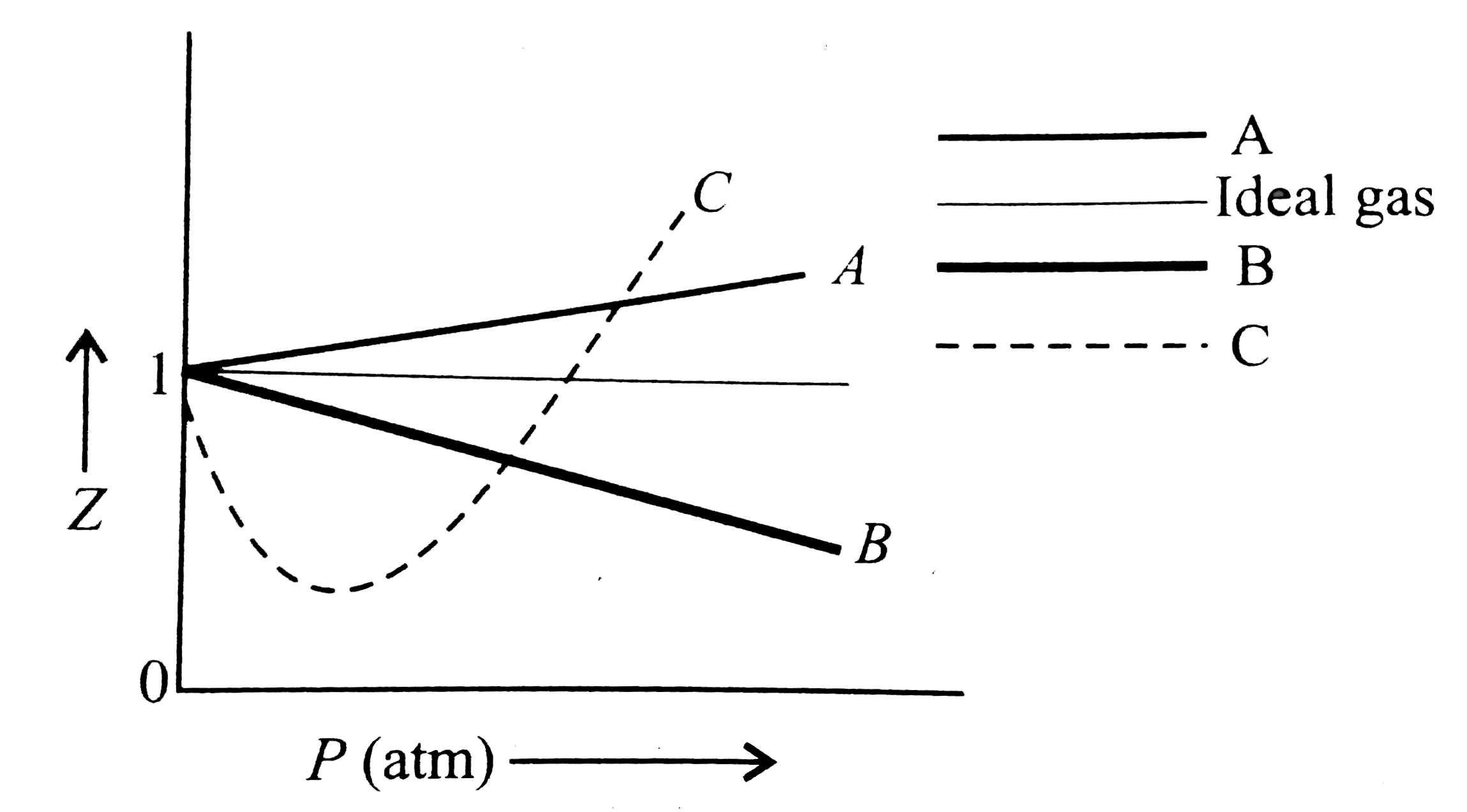
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12

Chem II - Real Gases: Van der Waals (Liquids and Solids)
