20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
$ 26.00 · 4.9 (761) · In stock
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

a) Compressibility factor Z obtained from the Lee-Kesler EoS, and

Objectives_template

Solved Problem 2. ( 30 points) The ideal gas law can

If Z is a compressibility factor, Van der Waals equation at low pressure can be written as

Solved Problem 2. ( 30 points) The ideal gas law can

Van der Waals equation, when pressure correction is ignored, one mole can be written as P(V - b) = RT. The correct expression compressibility factor will be

Solved The ideal gas law can represent the

If Z is a compressibility factor, van der Waals equation at low pressure ..

At low pressures For 1 mole, the van der Waals equation is written as [ p + a / V 2] V = RT The compressibility factor is then equal to:A. 1

If Z is a compressibility factor, van der Waal's equation low pressure can be written as : tot gnolaszemit sem st263 nisho ad Phim shuplamenu Pb (1) Z = 1 - (
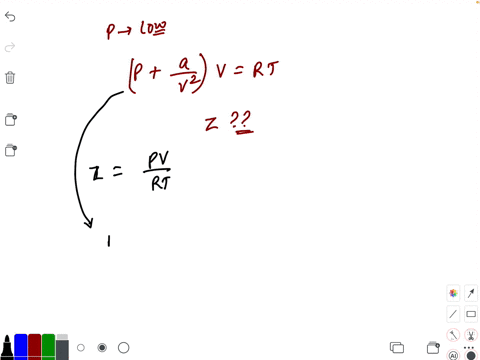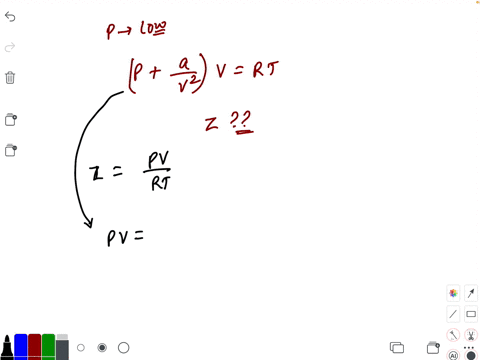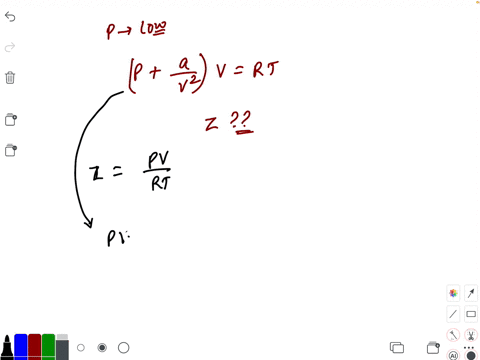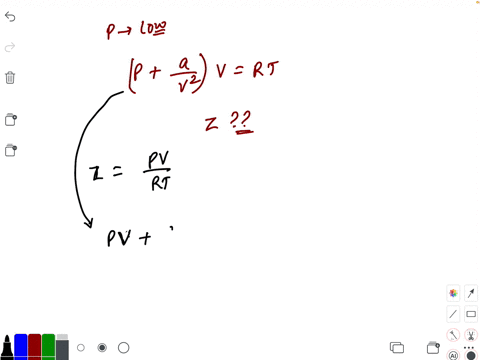
⏩SOLVED:At low pressures, van der Waals' equation is written as…
Solved 2. (20 points) At low pressures, the compressibility
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as

SOLUTION: Hssrptr plus one che 5 states of matter question answers - Studypool
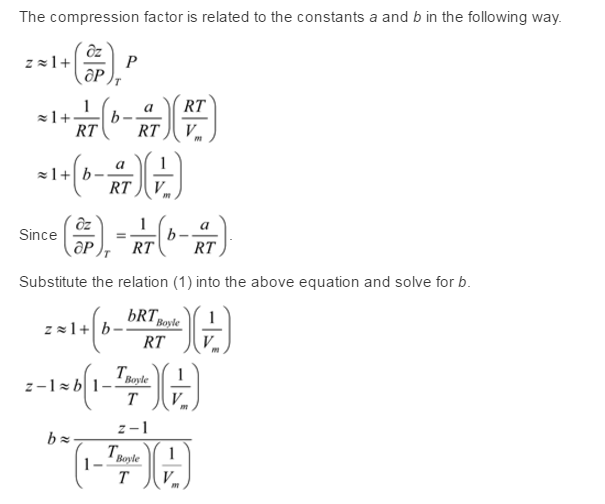
Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)
