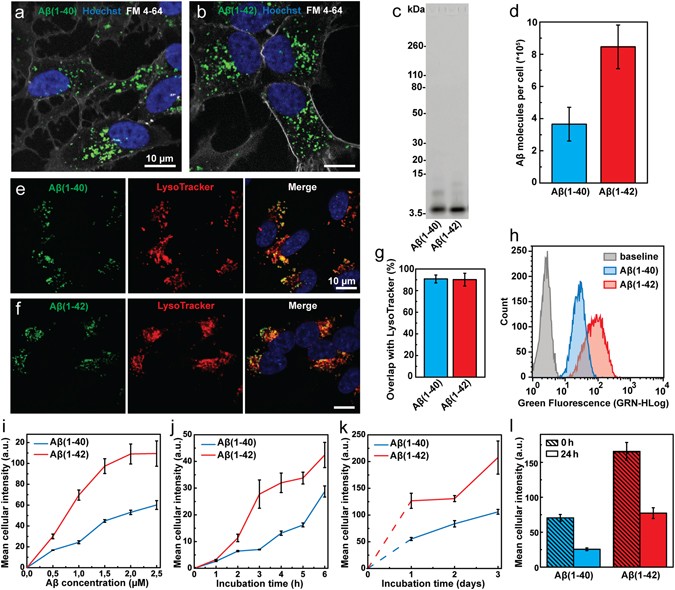
Endocytic uptake of monomeric amyloid-β peptides is clathrin- and dynamin-independent and results in selective accumulation of Aβ(1–42) compared to Aβ(1–40)
$ 6.50 · 5 (768) · In stock

In vivo synaptic activity-independent co-uptakes of amyloid β1–42 and Zn2+ into dentate granule cells in the normal brain

Evidence for aggregation-independent, PrPC-mediated Aβ cellular internalization. - Abstract - Europe PMC

Amyloids facilitate DNA transfection in vivo - ScienceDirect

Amyloid-beta peptides 40 and 42 employ distinct molecular pathways for cell entry and intracellular transit at the BBB endothelium

Human amyloid-β enriched extracts: evaluation of in vitro and in vivo internalization and molecular characterization, Alzheimer's Research & Therapy

Designed Cell-Penetrating Peptide Inhibitors of Amyloid-beta Aggregation and Cytotoxicity - ScienceDirect

Amyloid-beta peptides 40 and 42 employ distinct molecular pathways for cell entry and intracellular transit at the BBB endothelium

IJMS, Free Full-Text

The Amyloid Cascade Hypothesis 2.0 for Alzheimer's Disease and Aging-Associated Cognitive Decline: From Molecular Basis to Effective Therapy

Amyloids facilitate DNA transfection in vivo - ScienceDirect

Alzheimer's disease linked Aβ42 exerts product feedback inhibition on γ-secretase impairing downstream cell signaling