Co-processed materials testing as excipients to produce Orally
$ 34.99 · 4.8 (770) · In stock
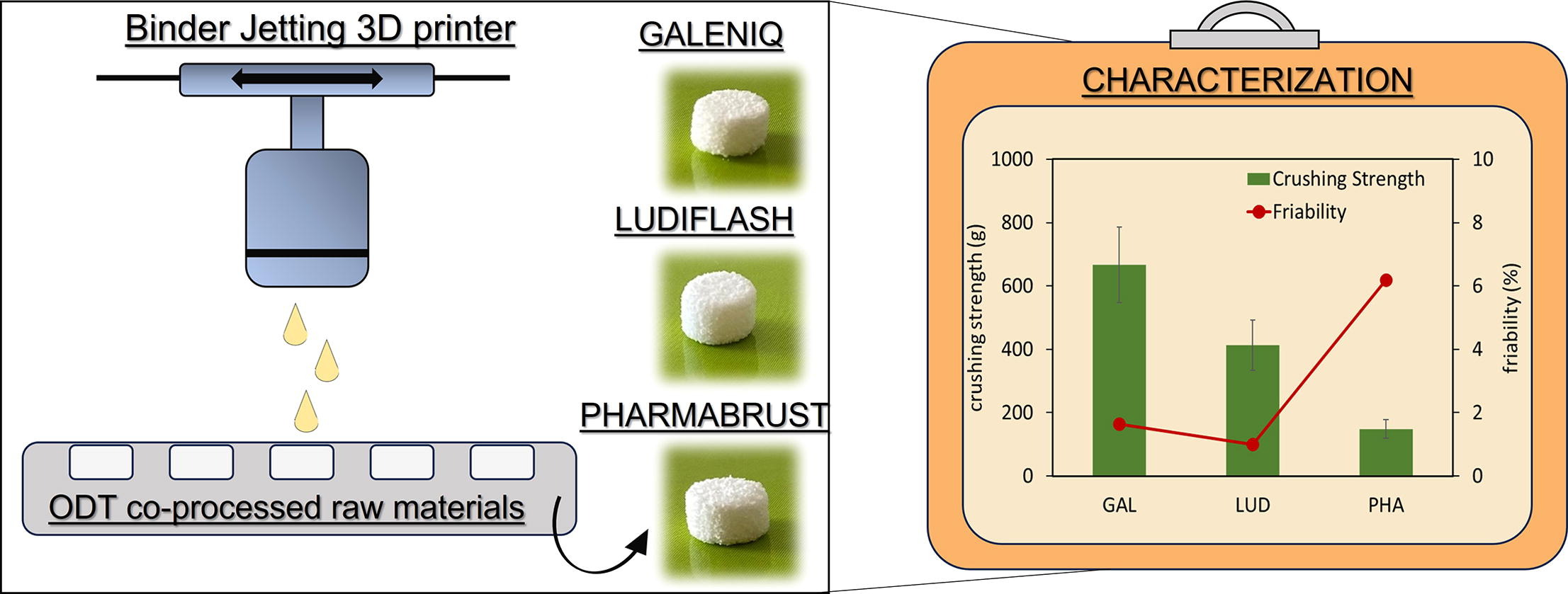
The study aimed to estimate the use of commercially available co-processed excipients, conventionally applied in compression protocols.
The use of co-processed materials for Orally Disintegrating Tablets (ODT) preparation by direct compression is well consolidated. However, the evaluation of their potential for ODT preparation by 3D printing technology remains almost unexplored. The

Co processed excipient

Pharmaceutical applications of powder-based binder jet 3D printing process – A review - ScienceDirect
Co-processed materials testing as excipients to produce Orally Disintegrating Tablets (ODT) using binder jet 3D-printing technology - Pharma Excipients

Co-processed materials testing as excipients to produce Orally Disintegrating Tablets (ODT) using binder jet 3D-printing technology - Pharma Excipients

Co-Processed Excipients for Dispersible Tablets—Part 2: Patient Acceptability

Co-processed materials testing as excipients to produce Orally Disintegrating Tablets (ODT) using binder jet 3D-printing technology - ScienceDirect

PDF) Innovative Color Jet 3D Printing of Levetiracetam Personalized Paediatric Preparations

New BENEO webinar: From powder to DC oral solid dosage forms - Pharma Excipients

Co-processed Excipients (CPE) from MEGGLE - Your best choice!
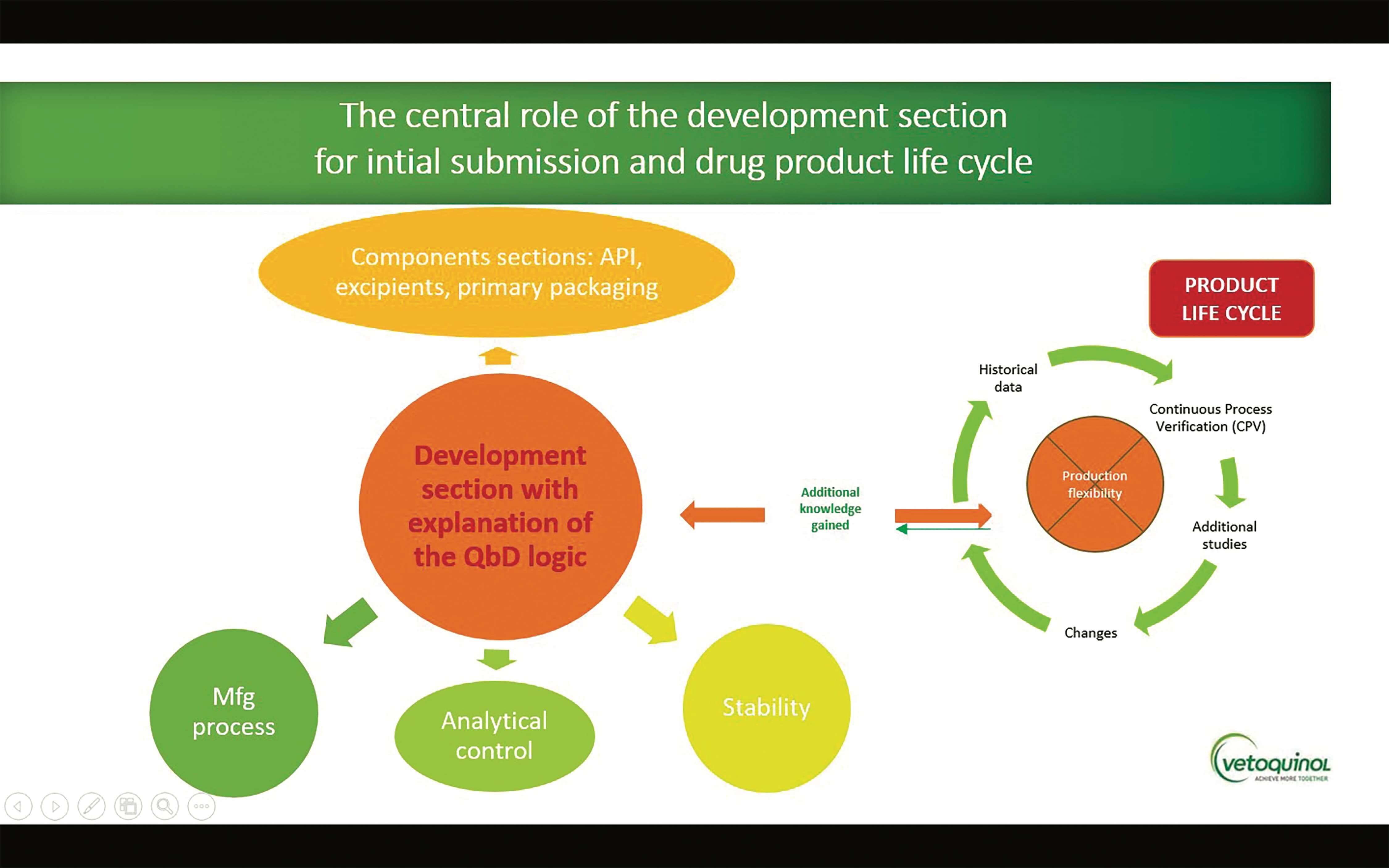
First steps towards ICH Q12: Leveraging process understanding & development data to define process Established Conditions - A3P - Industrie Pharmaceutique & Biotechnologie

Beneo - galenIQ - pharma excipients

Linda BARBIERI, PhD, Università degli Studi di Milano-Bicocca, Milan, UNIMIB, Department of Biotechnology and Biosciences

Co-Processed Excipients for Dispersible Tablets—Part 2: Patient Acceptability

Saliha MOUTAHARRIK, PostDoc Position, Bachelor of Industrial Pharmacy, University of Milan, Milan, UNIMI, Department of Pharmaceutical Sciences (DISFARM)