32. 80 g of h2 is reacted with 80 g of o2 to form water. find out
$ 16.50 · 4.6 (670) · In stock
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

How many grams of water are produced if we react 3 moles of
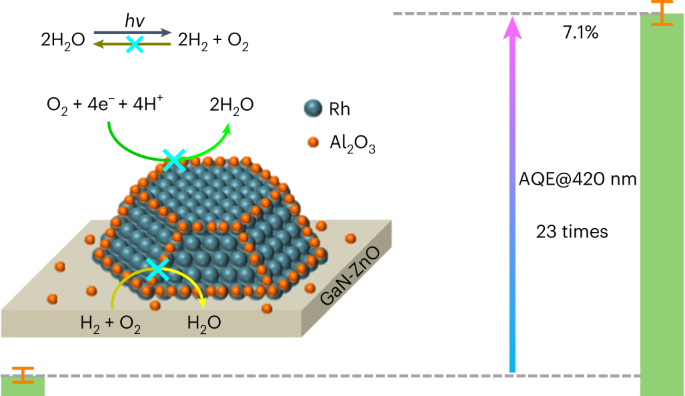
Blocking the reverse reactions of overall water splitting on a Rh
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting

SOLVED: Which is the limiting reactant when 5.00 g of H2 and 10.0
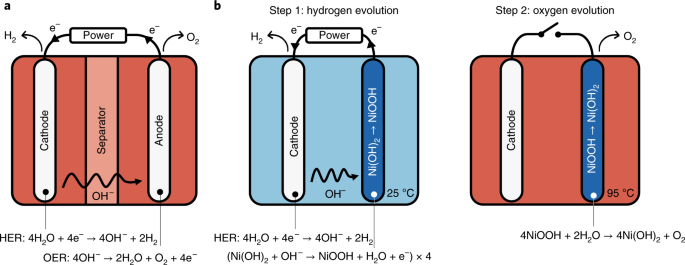
Decoupled hydrogen and oxygen evolution by a two-step
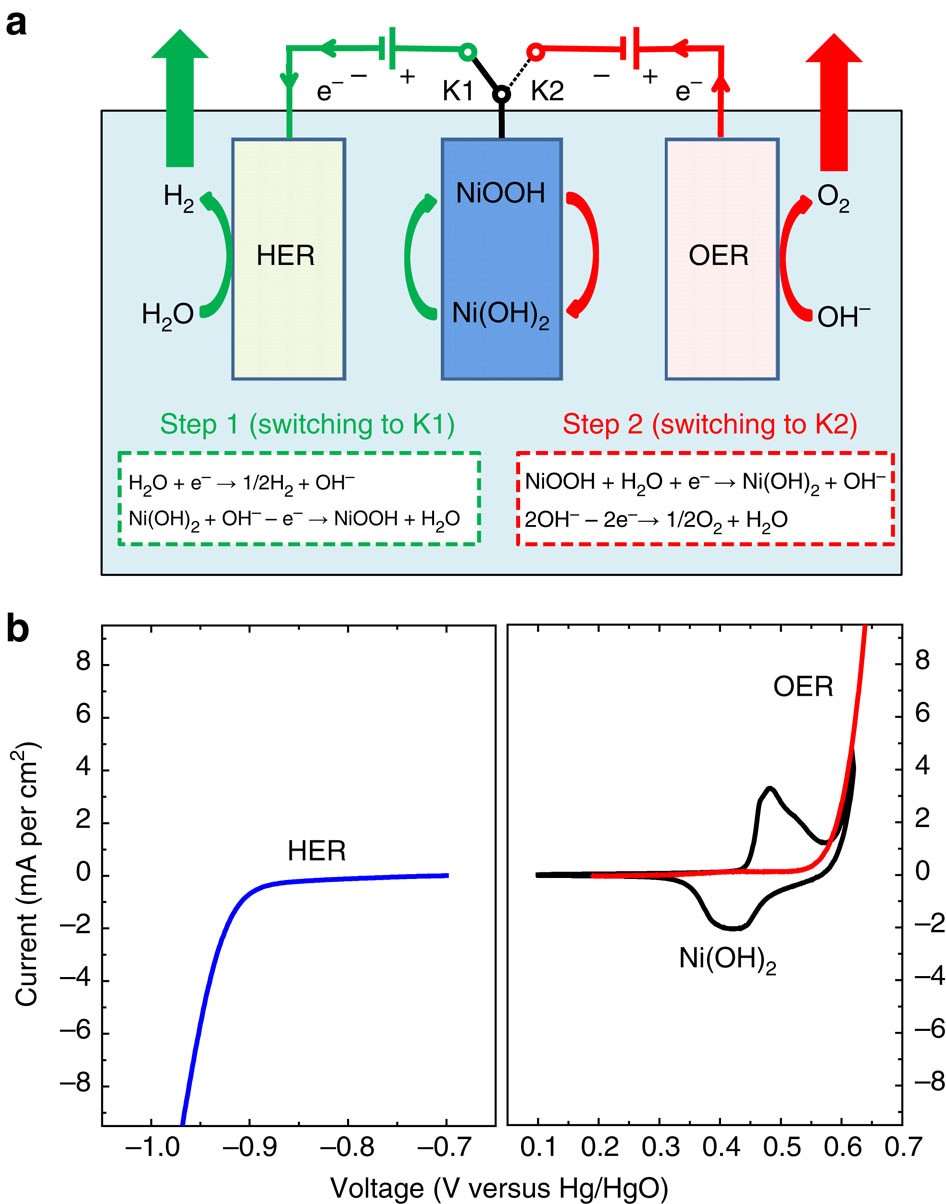
Separating hydrogen and oxygen evolution in alkaline water

A new high efficiency catalyst of Co–Ni/CeO2 for hydrogen

SOLVED: Question 1: CH4 + 2 H2O → 4 H2 + CO2 Given 80 g of CH4
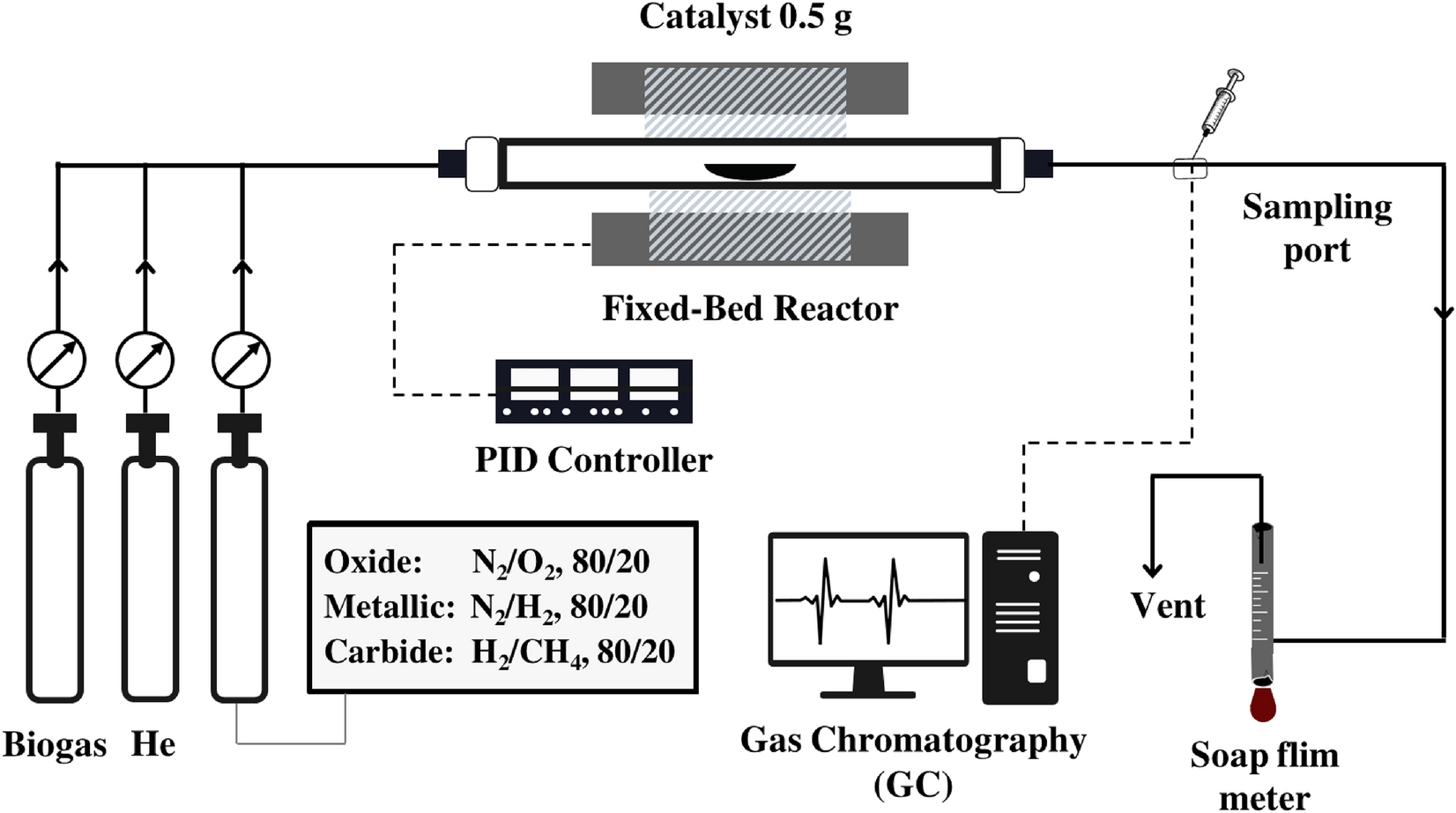
Development of Ni–Mo carbide catalyst for production of syngas and
How many grams of water can be produced if sufficient hydrogen
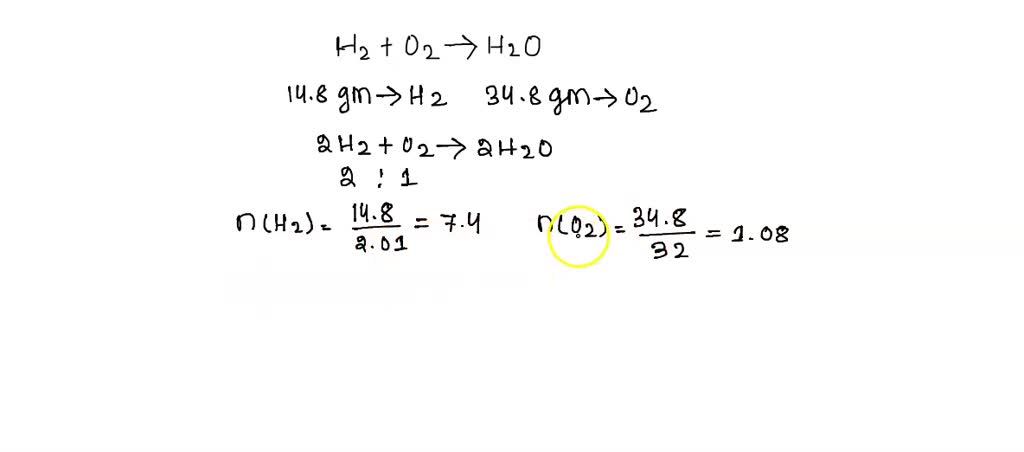
SOLVED: Question 5 Not yet answered Marked out of 1.00 Flag